
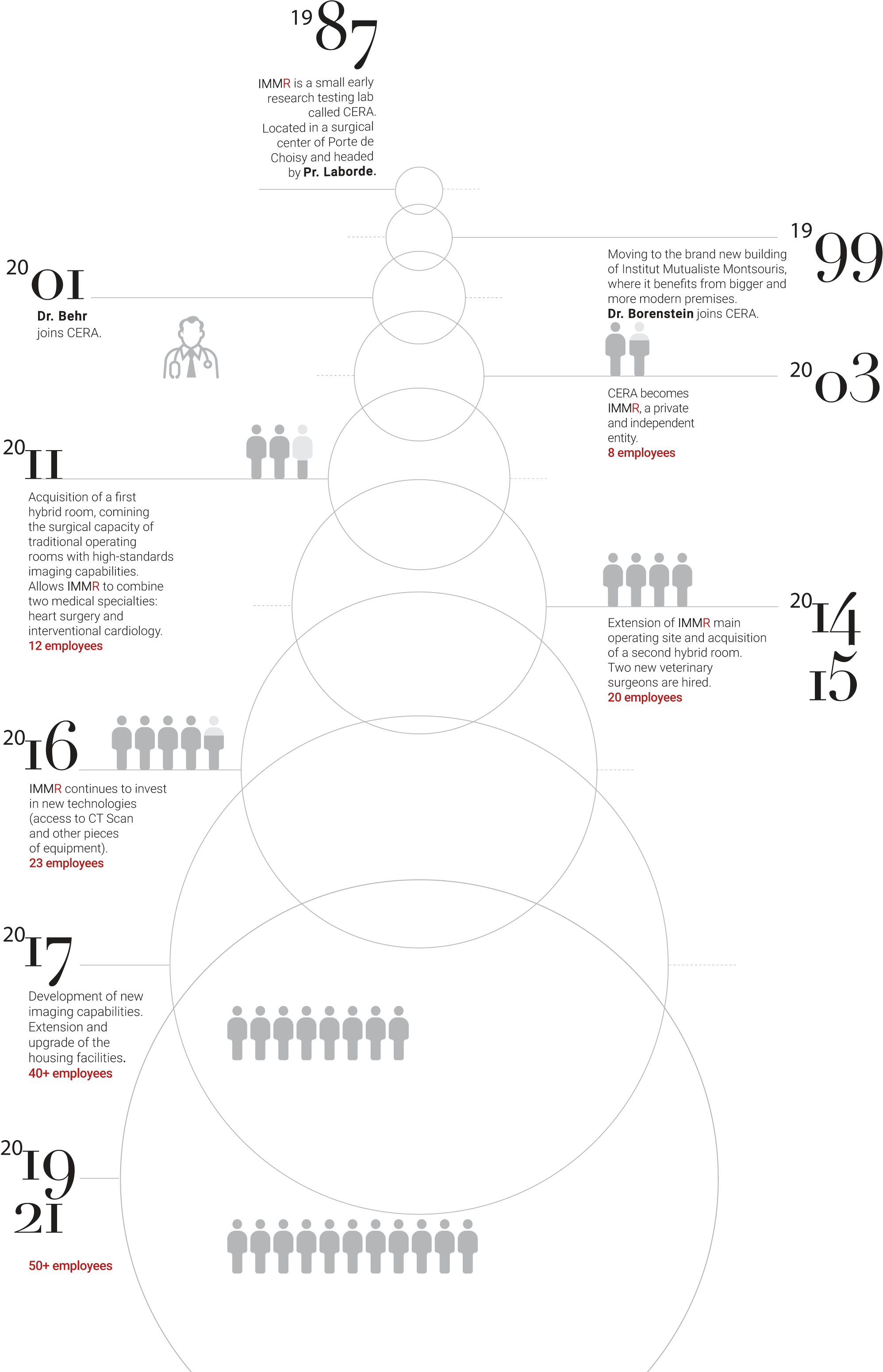
history
to readiness for human clinical trials.
30 Years of development to offer the best testing environment and capabilities.
our vision
delivered on time and on budget.
Your needs are our priorities.
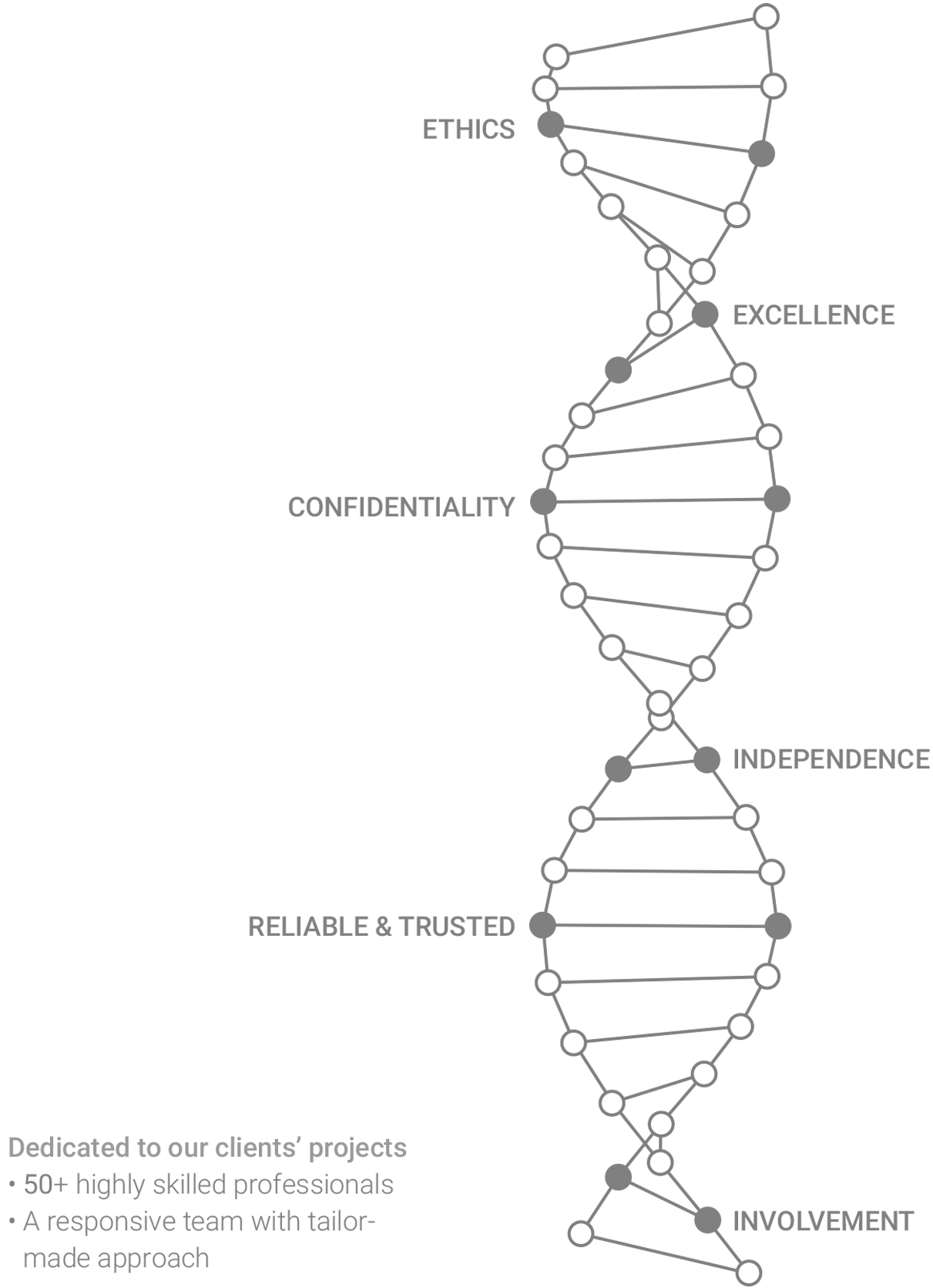
Our DNA
IMMR is renowned internationally as setting the standard for large models preclinical studies. We accelerate the development of fast-growing innovations, providing excellent services with world-class experts, cutting-edge equipment, state-of-the-art surgical suites and modern husbandry facilities. We are GLP-compliant and registered with the US FDA.
IMMR’s committed, 40+ people team (surgical experts, technicians, pathologists and QA professionals, among others) works with passion and high ethical standards, mastering procedures that insure low morbidity and mortality and high success rates, reducing time to market, costs and subjects utilization.IMMR guarantees work at the highest levels of integrity, and as our clients (industry leaders and start-ups alike), you can have absolute confidence in our lab, in the results and conclusions we provide to take your project to the next level. We believe that being independent and impartial allows us to offer the best services possible to all of our clients. We understand that as dedicated CEOs, R&D teams, clinicians and investors, you are working to develop important MedTech and Biotech innovations that will significantly advance patient care. By remaining objective and giving you unbiased opinion we can help you move forward with confidence. We assign to you any improvements we contribute and we never accept equity in any of our clients’ companies.
Finally, we also understand that confidentiality is critical. We have strict procedures to insure that your proprietary technology, results and intellectual property always remains safe.
OUR VALUES
at all levels of its organization.
- Providing unparalleled technical excellence in preclinical research.
- Providing outstanding customer service and 100% customer satisfaction.
- Making substantive contributions to medical innovation and human health.
- Maintaining the highest ethical standards and concern for animal welfare.

Device, delivery system and procedure design expertise.
- Shared free of charge
- Assignment of all new IP to the customer

- Early R&D acute studies
- Non GLP chronic studies
- GLP chronic studies
- Investigators
- Customers
- Field clinical specialists
- Rapid ACUC approvals
- Short procurement times
- Ability to schedule studies when needed
- Dedicated ethics committee
- Fully staffed and overseen by licensed veterinarians
> Anesthesia management
> Surgical technique
> Nursing care and husbandry
> Pathology
- Exceptional surgical outcomes minimizes animal utilization
- Husbandry facilities that meet or exceed AAALAC standards
- Services donated for treatment of companion animals

when our team
becomes your team.
our experts
Our world-class experts are client oriented, dedicated to the projects of our customers,
with more than 30 years of shared experience in surgery and research.
They are efficient and work with passion and commitment on MedTech and Biotech innovations,
and our clients come from all over the world to live a successful adventure with us!
OUR CHEMISTRY
Our team includes surgeons, veterinarians specialized in surgery and interventional radiology, imaging and pathology, quality assurance managers, research associates, histopathology specialists, medical writers, laboratory technicians, veterinary technicians and support staff. We are client oriented, and we accompany you from the early stage proof of concept to regulatory GLP studies for the FDA, cFDA or CE mark.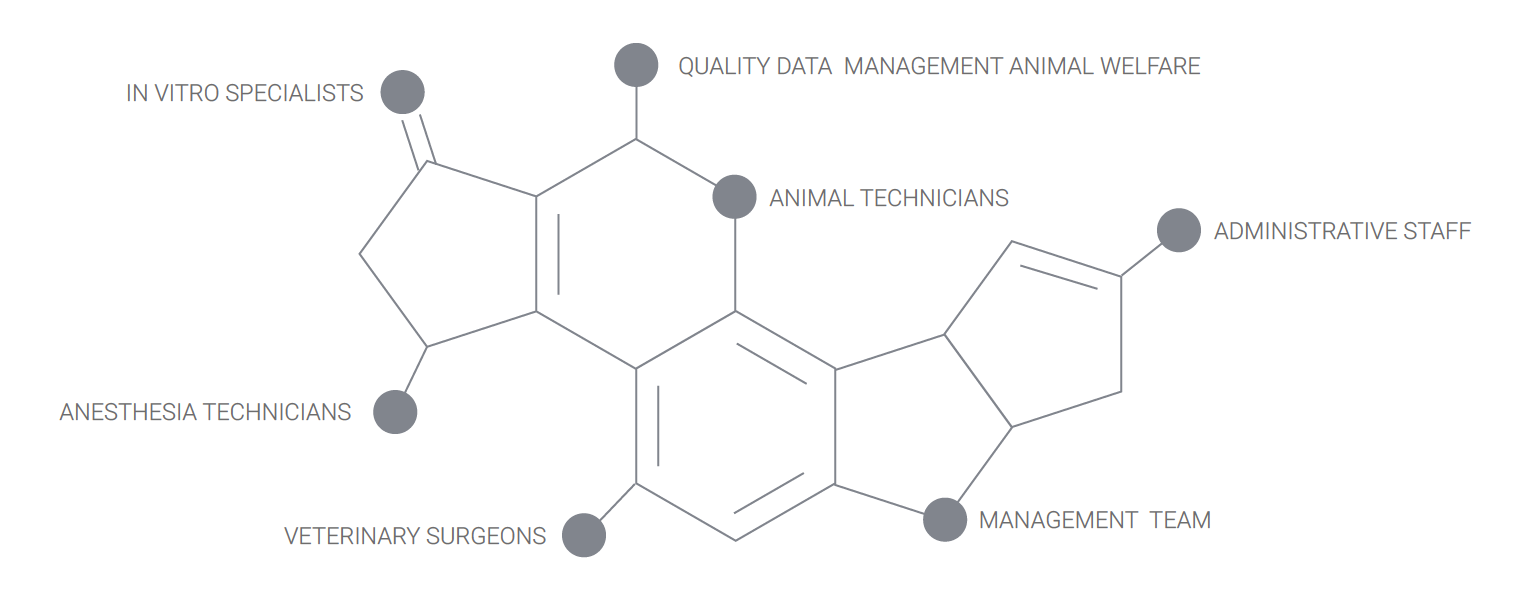
MANAGEMENT TEAM EXECUTIVE BOARD

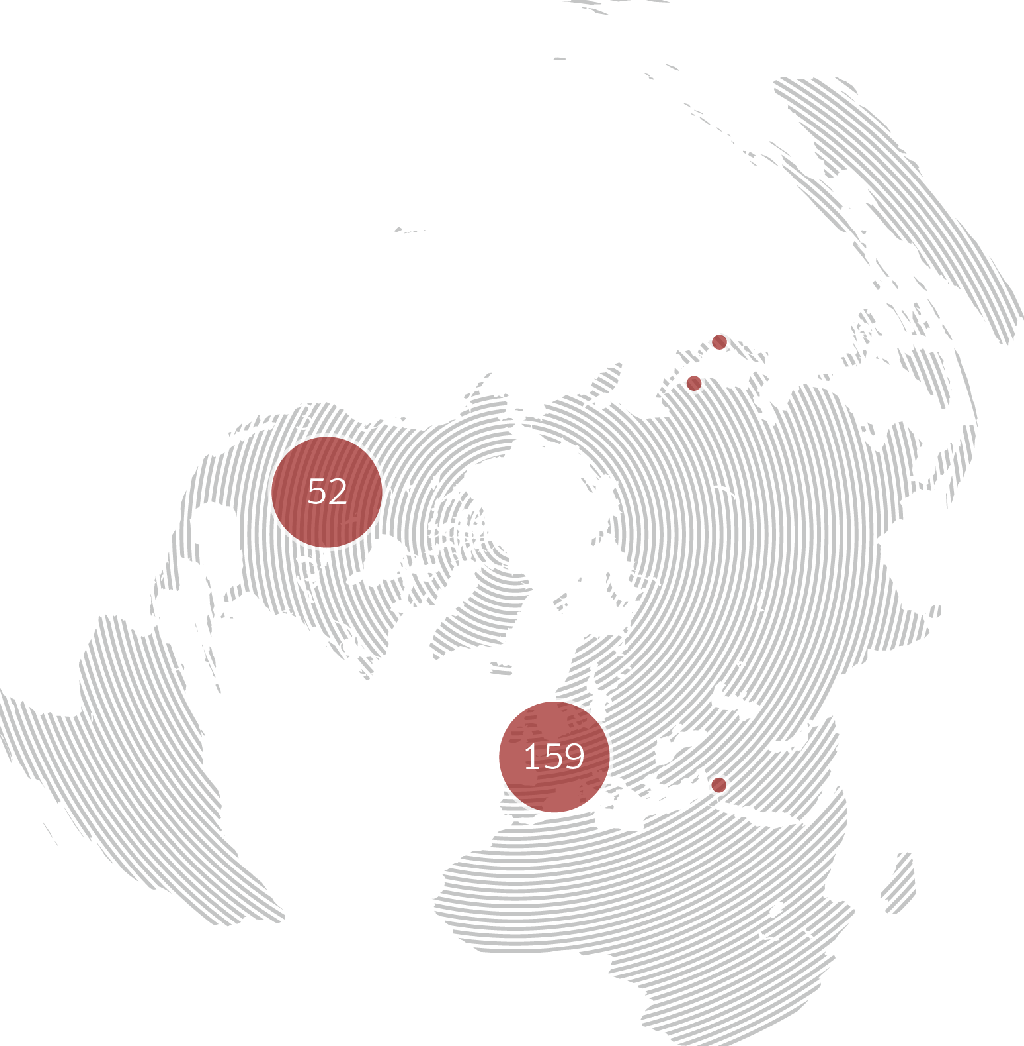
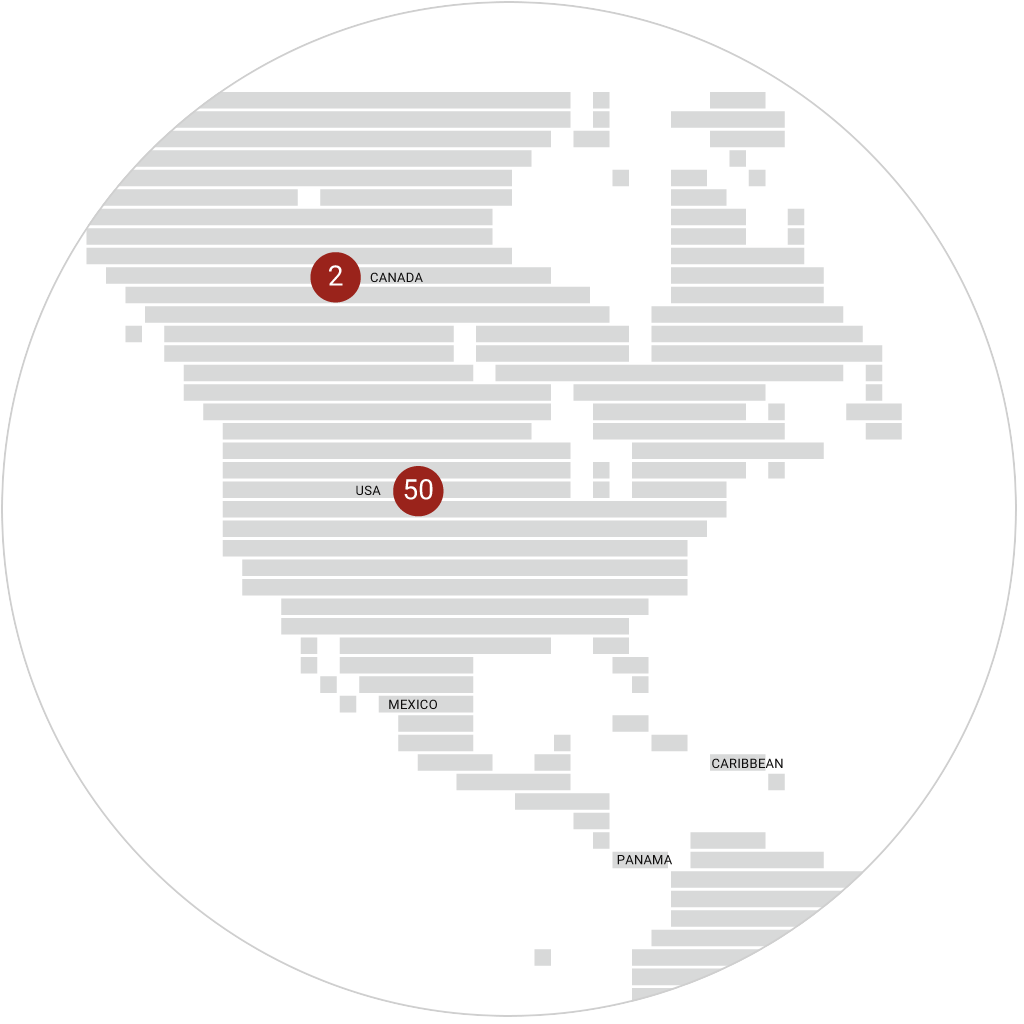
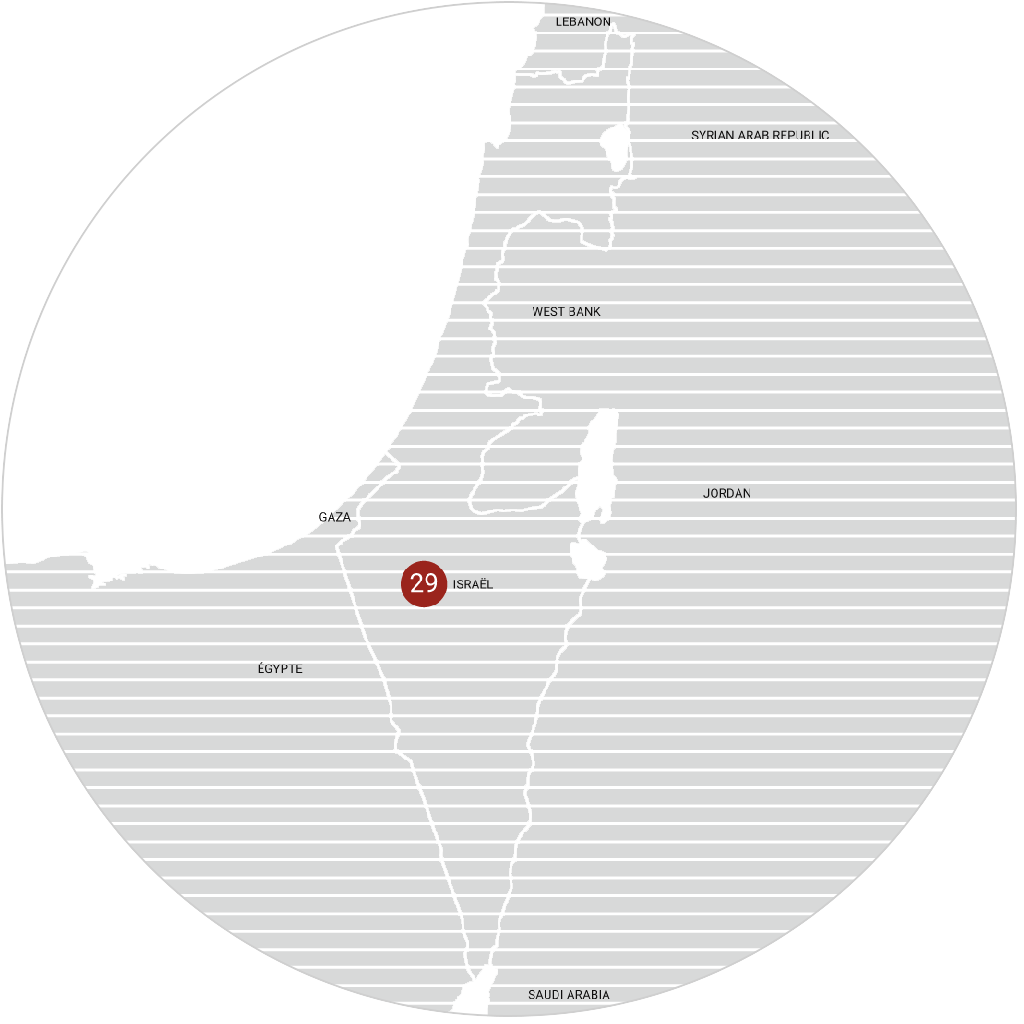
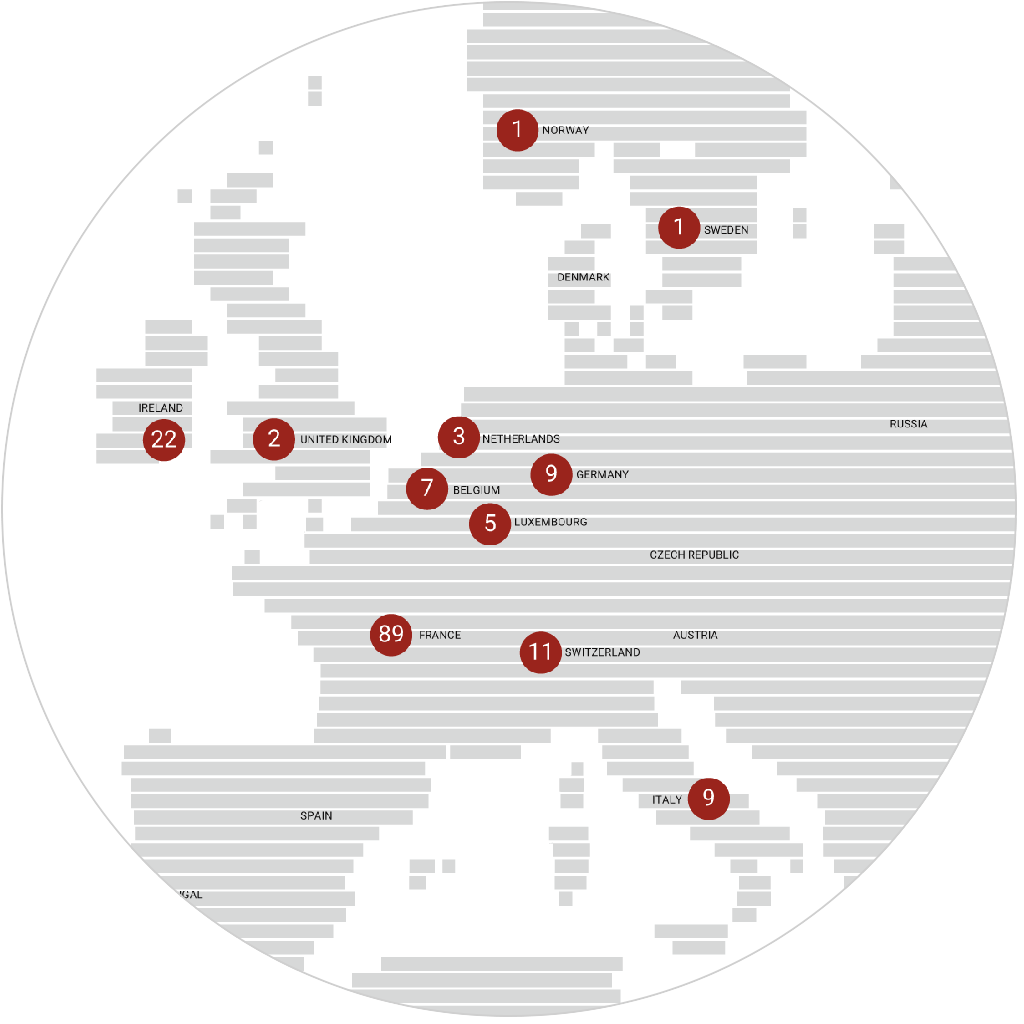
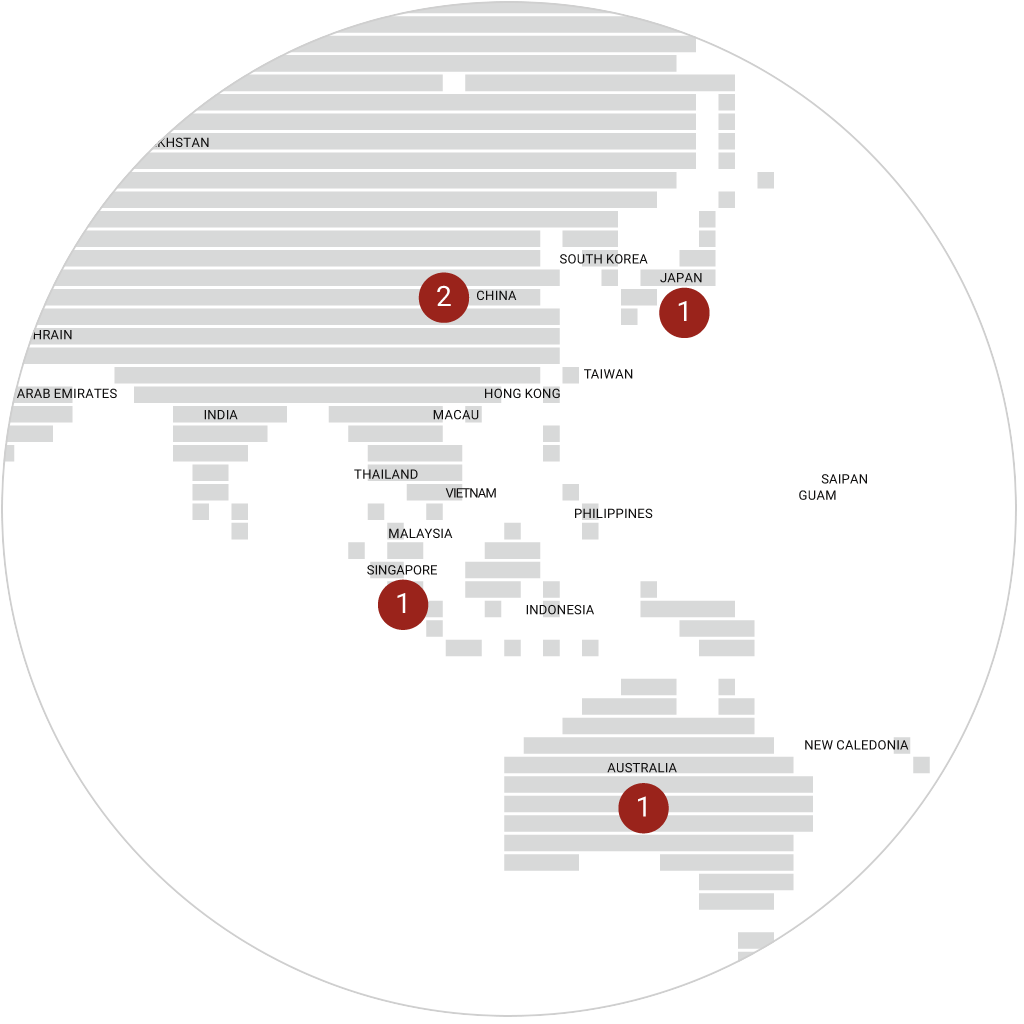
immr wordlwide
setting the standard for preclinical
studies in large models.
OUR PARTNERS






AMONGST MANY OTHERS, THEY TRUST US

























































